Despite the significant progress made in mRNA delivery based on classical cationic carriers, adverse events inevitably occur, including high inflammation and cellular toxicity, due to the need to compact mRNA through electrostatic interactions caused by the overabundance of cationic charge density in lipids. How to develop disruptive technologies to overcome the cationic properties of lipids is still a major challenge for safe and effective mRNA delivery.
Recently, a research paper titled “Biomimetic noncationic lipid nanoparticles for mRNA delivery” was published online on PNAS. In this study, the team prepared non-cationic thiourea lipid nanoparticles (NC-TNPs) that compress mRNA through the strong hydrogen bond interactions between the thiourea groups in NC-TNP and the phosphate groups in mRNA, dispensing with concealment and traditional electrostatic forces, and building mRNA-cationic lipid formulation.
NC-TNP is an mRNA delivery system that is simple, convenient, and repeatable in preparation and has no significant inflammation and cytotoxic side effects. Moreover, researchers found that NC-TNP can escape from the circulation route and inhibit the exocytosis of internalized nanoparticles from intracellular compartments to the extracellular environment, a common phenomenon in mRNA-LNP (lipid nanoparticle) formulations. Therefore, mRNA wrapped in NC-TNP showed a higher gene transfection efficiency both in vitro and in vivo compared to mRNA-LNP formulations. Surprisingly, compared to traditional LNPs, NC-TNP showed greater splenic targeting delivery capability and a higher accumulation ratio (spleen/liver). The spleen-targeting NC-TNP with mRNA as the target had a higher mRNA coding antigen expression in the spleen and induced a stronger immune response.
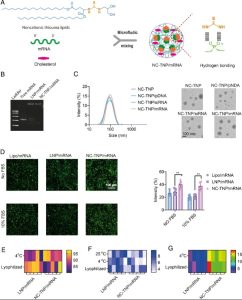
Figure 1. Design and characterization of noncationic mRNA delivery system. (Wang C,2023)
mRNA-based therapies are promising strategies for many biological and therapeutic applications, mainly including protein replacement therapies, immunotherapies for cancer or other diseases, and genome editing. In clinical applications, the use of naked mRNA in vivo is challenging due to the instability and susceptibility to degradation of extracellular RNases, and the inhibition of transmembrane transport by mRNA’s negative charge and large size (300~5000 kDa). Therefore, an appropriate delivery system is needed to unleash the potential of mRNA drugs for disease treatment. The mRNA delivery systems currently used to envelop endonuclease for protection and transportation to the intracellular compartments focus on using four-component LNPs containing cationic lipids or ionizable lipids, phospholipids, cholesterol, and PEG lipids.
mRNA delivery vehicles of Creative Biolabs:
| Lipid-based vectors | Lipoplex |
| Lipid nanoparticle | |
| Polymer-based vectors | Polyplex |
| Micelleplex | |
| Hybrid vectors | Lipopolyplexes |
| Cationic Nanoemulsions |
LNPs also have limitations, including a complex four-component preparation process, adverse events of high inflammation and cellular toxicity, suboptimal internalization into the cytoplasm and low gene transfection efficiency due to expulsion from the body through circulatory pathways, and unintended targeting to specific tissues other than the liver. These limitations are primarily caused by the excess cationic charge density of lipids that needs to be compressed by electrostatic interaction with mRNA. The conflicting cationic charge requirements of mRNA delivery systems put the development of LNPs in a bind. How to develop disruptive technology and discard bounds and traditional electrostatic forces to construct mRNA-cationic lipid formulations presents a significant challenge for safe and effective mRNA delivery.
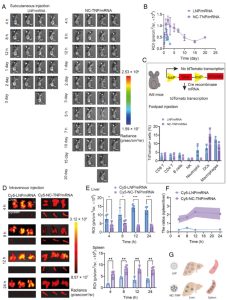
Figure 2. In vivo mRNA transfection and targeted delivery of NC-TNP/mRNA to spleen. (Wang C,2023)
Based on this, the study incorporated a thiourea group as a hydrogen bond donor into lipids, preparing a non-ionic thiourea lipid nanoparticle (NC-TNP) with T-type hydroxyl as an auxiliary bond donor. The strong hydrogen bond interaction between the thiourea group in NC-TNP and the phosphate group in mRNA is used for complexation with mRNA, abandoning the traditional electrostatic force, and constructing the mRNA-cationic lipid formulation. In vitro gel retardation experiments showed that the non-ionic NC-TNP can effectively encapsulate siRNA, pDNA and mRNA, thus providing long-lasting protection against nuclease degradation, highlighting the multifunctionality of this method. Notably, compared with cationic LNP, NC-TNP, as a simple delivery system, shows a higher mRNA transfection efficiency both in vitro and in vivo. This is due to NC-TNP’s ability to escape the circulatory path, inhibiting the excretion of internalized nanoparticles from the cell to the extracellular environment, a common phenomenon in mRNA-LNP preparation.
Unlike cationic or ionizable mRNA-LNP nanomedicines with positive charge safety, NC-TNP demonstrates negligible inflammation and cytotoxicity adverse reactions in vivo. Surprisingly, in vivo distribution and mRNA transfection results showed that, compared with traditional mRNA-LNP preparations, mRNA wrapped in NC-TNP can selectively transport to spleen tissue, with a higher accumulation ratio (spleen/liver) and stronger mRNA-encoded protein expression. Compared with the LNP platform, the intravenous mRNA delivery induced by the NC-TNP system led to a higher mRNA-encoded antigen-specific CD8+ T cell and comparable antibody responses.
In summary, this work provides evidence for the concept of constructing a non-ionic mRNA delivery system with good inflammation safety, high gene transfection efficiency, and splenic targeting delivery to induce long-lasting and robust humoral and cell-mediated immunity for disease treatment.
Reference
Wang C, Zhao C, Wang W, et al. Biomimetic noncationic lipid nanoparticles for mRNA delivery[J]. Proceedings of the National Academy of Sciences, 2023, 120(51): e2311276120.
