RNA therapy is a novel therapeutic strategy that leverages RNA molecules to regulate gene expression or induce immune responses. RNA therapy possesses several advantages, such as programmability, diversity, flexibility, and safety, and can be used to treat a variety of diseases, such as cancer, genetic diseases, infectious diseases, and autoimmune diseases. In recent years, significant breakthroughs have been made in the advancement of RNA therapy, particularly in the development and application of the COVID-19 vaccine, demonstrating the huge potential and prospects of RNA therapy.
However, RNA therapy also confronts several challenges and limitations, mainly the instability of RNA molecules, their immunogenicity, and inefficient cellular uptake. To overcome these obstacles, researchers have developed various methods to chemically modify RNA to increase its stability, reduce its immunogenicity, and enhance its transcription efficiency. The primary methods for chemically modifying RNA include modifying the caps, tails, nucleotides, and structures of RNA, as well as constructing RNA hybrids and circular RNAs. These methods can be optimized and combined for different types of RNA and target diseases to achieve the best therapeutic effects of RNA.
Recently, an article titled “Chemically Modified Platforms for Better RNA Therapeutics” was published in the Chemical Review by Tao Wei and Kong Na from Harvard Medical School, and Liu Gang from Xiamen University, among others. The article summarizes the latest advancements in chemically modified RNA therapeutic platforms, focusing on the various methods of chemically modifying RNA, their mechanisms, advantages, disadvantages, and applications. Moreover, it also discusses the future direction and challenges of chemically modified RNA therapeutic platforms with the aim of providing beneficial references and insights for research and clinical applications of RNA therapy.
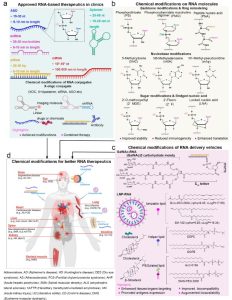
Fig.1 Chemical modifications promote better RNA clinical applications (Shi Y, 2024)
Types and principles of RNA therapy
RNA therapy is a novel treatment strategy that uses RNA molecules to regulate gene expression or induce immune responses. RNA molecules are fundamental components of life, involved in many crucial biological processes such as transcription, translation, splicing, silencing, and regulation. The types and functions of RNA molecules are very diverse, and can be categorized into coding RNAs and non-coding RNAs. Coding RNAs mainly include messenger RNA (mRNA), responsible for carrying genetic information from DNA to proteins. Non-coding RNAs include transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), microRNAs (miRNA), small interfering RNA (siRNA), and long non-coding RNA (lncRNA), which are responsible for RNA processing, transport, stability, degradation, and regulation.
The principle of RNA therapy is to deliver exogenous RNA molecules into cells to alter intracellular RNA levels or functions, thereby achieving modulation of gene expression or immune responses. Based on the type and mode of action of RNA molecules, RNA therapies can be divided into the following types:
- mRNA therapies
mRNA therapy is a strategy that uses exogenous mRNA molecules to express target proteins. It can be used to replace missing or abnormal proteins, such as enzymes, receptors, or factors, or to express therapeutic proteins, such as antibodies, vaccines, and drugs. The advantages of mRNA therapy include avoiding the risk of DNA integration, flexible regulation of protein expression level and timing, being applicable to various cell types and organ tissues, and being able to elicit strong immune responses.
- siRNA therapies
siRNA therapy is a strategy that uses exogenous siRNA molecules to silence target genes. siRNA molecules are double-stranded RNA molecules, approximately 21–23 nucleotides in length, that can bind complementary sequences of target mRNA molecules, leading to mRNA degradation or translational inhibition. siRNA therapy can be used to silence overexpressed or abnormal genes such as oncogenes, viral genes, mutant genes, or genes associated with disease, such as inflammation genes, fibrinolytic genes, and coagulation genes. The advantages of siRNA therapy include specifically silencing target genes, effectively reducing gene expression levels, and reversing abnormal genetic states.
- miRNA therapies
miRNA therapy is a strategy that uses exogenous miRNA molecules or their antagonists to regulate target genes. miRNA molecules are single-stranded RNA molecules, approximately 22 nucleotides long, that can bind partially complementary sequences of target mRNA molecules, leading to mRNA degradation or translational inhibitions. miRNA molecules are crucial gene regulatory factors involved in many physiological and pathological processes such as cell differentiation, proliferation, apoptosis, metabolism, immunity, and carcinogenesis. miRNA therapy can be used to increase or reduce miRNA levels, thereby regulating miRNA target genes and promoting or inhibiting cell growth, death, migration, invasion, and so on. The advantages of miRNA therapy include simultaneously regulating multiple genes, affecting cell fate and function, and being applicable to various diseases.
The method and mechanism of chemical mdifications of RNA
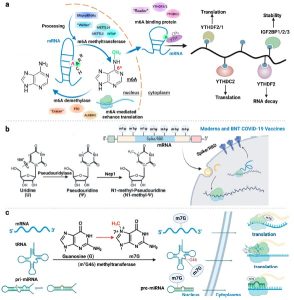
Fig.2 Nucleobase modification (Shi Y, 2024)
In order to overcome the challenges and limitations of RNA therapy, researchers have developed various methods of chemical modification of RNA, aiming to enhance RNA stability, reduce RNA immunogenicity, and enhance the efficiency of RNA transcription. The methods for chemically modifying RNA mainly include modifications to the cap, tail, nucleotide, and structure of the RNA, as well as the construction of RNA hybrids and cyclic bodies. These methods can be optimized and combined based on different types of RNA and target diseases to achieve the most optimal RNA therapy effects.
The RNA cap is a unique structure located at the 5′ end of the RNA, composed of a 7-methylguanylate and one to three methylated diphosphates. The RNA cap protects RNA from degradation by external enzymes, promotes the nuclear export and transport of RNA, enhances RNA translation efficiency, and regulates RNA splicing and silencing. However, it can also be recognized and removed by cellular enzymes, resulting in the instability and immunogenicity of the RNA. Therefore, researchers have chemically modified the RNA cap to enhance its stability and compatibility, for example, by replacing the 7-methylguanosine of the RNA cap with other nucleotides such as 2-thioguanosine, 2-oxyguanosine, and 2-fluoroguanosine. This can reduce the immunogenicity of the RNA cap, increase its stability, and improve the RNA translation efficiency. Other functional groups can further enhance the stability and compatibility of the RNA cap.
Our mRNA Capping Services
RNA tail modification
The RNA tail, a common structure at the 3′ end of the RNA, is made up of a string of polyadenylate (poly(A)). The RNA tail also plays several roles, such as protecting RNA from external enzyme degradation, promoting RNA nuclear export and transport, enhancing RNA translation efficiency, and regulating RNA stability and degradation. However, the RNA tail can also be recognized and degraded by cellular enzymes, which can lead to instability and immunogenicity of the RNA. Therefore, researchers have chemically modified the RNA tail to enhance its stability and compatibility. This can be achieved in a number of ways, including by replacing the adenosine in the RNA tail with other nucleotides such as guanosine, cytosine, and uridine. This can reduce the immunogenicity of the RNA tail, increase its stability, and improve RNA translation efficiency.
Nucleotide modification of RNA
A nucleotide, the basic unit of RNA, consists of a nitrogenous base and a sugar component. There are four types of RNA nucleotides: adenine (A), guanine (G), cytosine (C), and uridine (U). The nucleotides of RNA determine its sequence and structure, which in turn influence the function and stability of the RNA. However, the nucleotides of RNA can also be recognized and degraded by cellular enzymes, which results in RNA instability and immunogenicity. Therefore, researchers have chemically modified the nucleotides of RNA to enhance their stability and compatibility.
Structural modification of RNA
RNA structure is the spatial conformation of the RNA, determined by its sequence and secondary, tertiary, and quaternary structures. This influences the function and stability of the RNA, including RNA folding, binding, catalysis, and recognition. Thus, researchers have used various chemical modifications to increase the stability and compatibility of the RNA structure.
Hybrid and cyclic RNA
Hybrids and cyclic forms of RNA are special RNA structures, the result of RNA combining with other molecules or self-assembling into complexes or closed structures. These have various functions such as enhancing RNA stability, reducing RNA immunogenicity, enhancing RNA transcription efficiency, and regulating RNA function and activity.
Chemical modification of the RNA treatment platform still faces several challenges and limitations that require further research and optimization, such as RNA design, synthesis, characterization, delivery, evaluation, etc. Methods of chemically modifying RNA need to be customized and combined according to different RNA types and target diseases to achieve higher specificity and multitasking. The application of chemically modified RNA needs more animal and human trials and verifications to prove its safety and effectiveness. The chemically modified RNA treatment platform needs to be synergistically integrated with other treatment strategies to achieve better therapeutic effects and a wider treatment range.
Reference:
Shi Y, Zhen X, Zhang Y, et al. Chemically Modified Platforms for Better RNA Therapeutics[J]. Chemical Reviews, 2024.
