In the ongoing battle against AIDS, scientists are relentlessly searching for a definitive cure. The recent and rapid advancements in mRNA technology, highlighted by the success of COVID-19 vaccines and the clinical application of gene-editing, have opened unprecedented opportunities in gene therapy and reshaped our understanding of life sciences.
Now, a new study focused on reversing HIV latency is pushing the boundaries of this field even further. Imagine if we could precisely “awaken” the HIV virus hiding within resting T cells, allowing us to eliminate these “latent enemies” once and for all. A true cure for AIDS might no longer be a distant dream.
A recent paper published in the international journal Nature Communications, titled “Efficient mRNA delivery to resting T cells to reverse HIV latency,” details how scientists from the University of Melbourne and other institutions are exploring mRNA lipid nanoparticles (LNP) as a new weapon against HIV latency. The latent state of HIV is the single greatest obstacle to a cure; while traditional antiretroviral therapy (ART) can effectively suppress viral replication, it cannot eliminate the latent viral reservoir. This has made the search for a new strategy—one that can activate latent HIV transcription and clear infected cells—a primary focus of research.
In this study, researchers developed a highly efficient mRNA-LNP delivery system that introduces mRNA into resting T cells to reverse HIV latency, offering new possibilities for a cure. The team targeted resting CD4+ T cells, the main reservoir for latent HIV, using an innovative LNP formulation called LNP X. This formulation, composed of the ionizable lipid SM-102 and β-sitosterol, demonstrated unprecedented transfection efficiency.
Development of an Innovative Delivery System
The research team began by optimizing the composition of the lipid nanoparticles (LNPs). They replaced the ionizable lipid DLin-MC3-DMA, found in the traditional patisiran LNP, with SM-102 and incorporated β-sitosterol, a natural analog of cholesterol, to create LNP X. This novel lipid combination significantly enhanced mRNA delivery efficiency while reducing cytotoxicity. Experimental results showed that LNP X achieved a remarkable 76% transfection efficiency in resting CD4+ T cells without prior stimulation, far surpassing the 2.1% efficiency of the traditional patisiran LNP. Furthermore, in stimulated CD4+ T cells, LNP X maintained a high efficiency of over 75%, even at low doses.
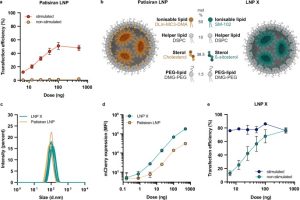
Fig.1 Without pre-stimulation, LNP X can effectively transfect primary CD4 + T cells.1
Experimental Validation: Reversing HIV Latency
To test LNP X’s ability to activate HIV transcription, researchers encapsulated mRNA encoding the HIV Tat protein within it. The Tat protein is a critical regulator of HIV transcription that promotes the synthesis of viral RNA. The results were clear: in CD4+ T cells treated with Tat-LNP X, the expression of HIV-related genes increased significantly. HIV RNA transcripts at all stages—including initiation, elongation, completion, and splicing—were markedly upregulated. In contrast, the control group, treated with RFP-LNP X, showed no such effect. The Tat-LNP X also induced the release of HIV viral particles, demonstrating its effectiveness in reversing HIV latency.
Potential for Delivering Gene-editing Activation Systems
Beyond the Tat protein mRNA, the researchers also successfully used LNP X to package and deliver a gene-editing activation system. This system consists of a catalytically inactive gene-editing protein fused to a transcriptional activation domain, which can specifically activate transcription at the HIV promoter region. The experiment confirmed that LNP X could successfully deliver the gene-editing system and induce the expression of HIV-related genes in CD4+ T cells. As expected, HIV RNA expression was significantly increased in cells treated with the gene-editing-LNP X, an effect not observed in the control group treated with a scrambled gene-editing-LNP X.
Limitations and Future Outlook
Although LNP X has shown immense potential for reversing HIV latency, the study acknowledges certain limitations. For instance, a single dose of Tat-LNP X, while significantly boosting HIV transcription, did not lead to virus-mediated cytotoxicity or immune-mediated clearance of the infected cells. This suggests that additional interventions may be required to either sensitize the infected cells to death or to promote their elimination. Furthermore, key properties of LNP X, such as its in vivo immunogenicity, biodistribution, and half-life, require further investigation to determine optimal therapeutic dosages and ensure safety.
In summary, this research provides a novel strategy for reversing HIV latency through the development of a highly efficient mRNA-LNP delivery system, LNP X. This platform not only delivers therapeutic mRNA to resting CD4+ T cells with high efficiency but also demonstrates significant potential for delivering other complex RNA-based therapies. With further research and optimization, there is strong reason to believe that this technology could lead to a new breakthrough in the quest for an AIDS cure, ensuring the “latent enemy” has nowhere left to hide.
At Creative Biolabs, we are dedicated to advancing your mRNA research and therapeutic programs. We provide a comprehensive suite of services, and our team specializes in creating custom mRNA delivery vehicles designed for maximum efficacy and safety.
We understand that effective delivery is key to your success. That’s why we offer specialized solutions to protect your mRNA payload and ensure it reaches its target efficiently. Our core service features include:
- Advanced Lipid-Based Vectors: We excel in designing and producing state-of-the-art lipid nanoparticle (LNP) systems. We utilize cutting-edge ionizable lipids to enhance endosomal escape and reduce toxicity, directly improving the therapeutic window of your mRNA drug.
- Versatile Polymer-Based Vectors: Leveraging the chemical flexibility of polymers, we build custom polyplexes and micelleplexes. These vehicles offer superior protection for your mRNA against degradation while ensuring efficient cellular uptake.
- Innovative Hybrid Vectors: We combine the best of both worlds by creating novel hybrid nanoparticles. By integrating materials like lipids and polymers (e.g., PLGA), we engineer highly stable and effective systems tailored to your specific delivery challenges.
Reference
- Cevaal, Paula M., et al. “Efficient mRNA delivery to resting T cells to reverse HIV latency.” Nature Communications1 (2025): 4979. CC BY 4.0. https://doi.org/10.1038/s41467-025-60001-2
