The era of messenger RNA (mRNA) has arrived, transforming the landscape of vaccines and therapeutics. The rapid development and success of mRNA vaccines have been a landmark achievement in modern medicine. However, this success was not built on the mRNA molecule alone. The true unsung hero of this story is the delivery vehicle: the Lipid Nanoparticle (LNP).
An LNP is a microscopic sphere of lipids designed to do one of the most difficult jobs in pharmacology: protect a fragile mRNA molecule, escort it past the body’s defenses, and deliver it inside the correct cells to be translated into a protein.
But this delivery process creates a fundamental challenge. To be effective, an LNP-based vaccine must be “seen” by the immune system to generate a powerful response—a quality known as immunogenicity. Yet, if it’s “seen” too aggressively, it can trigger excessive inflammation and side effects—a state known as reactogenicity.
For any company operating at the forefront of mRNA-based medicine, the core question is this: How do we design an LNP that achieves the perfect balance? How do we maximize the therapeutic good (immunity) while minimizing the bad (reactogenicity)?
Recent research, including data from our partners, sheds light on this complex balancing act. It’s not about finding one “magic bullet” component but about the meticulous optimization and tuning of the entire lipid formulation.
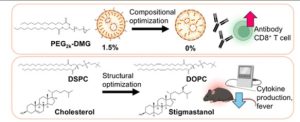
Fig.1 Optimizing mRNA-LNP lipids for better vaccines.1
Understanding the LNP: A Four-Part System
Think of an LNP as a high-performance vehicle. It’s not a single entity but an assembly of four critical components, each with a specific function. The precise ratio and chemical identity of these components determine the LNP’s size, charge, stability, and, ultimately, its in vivo biological performance.
- The Ionizable Lipid: This is the LNP’s engine. At an acidic pH (during manufacturing), it’s positively charged, allowing it to bind and encapsulate the negatively charged mRNA. At the body’s neutral pH, it becomes neutral, helping the LNP remain stable in circulation. Its primary role is to get the mRNA into the cell.
- The Helper Lipid (Phospholipid): This forms the structural “body” of the nanoparticle. It provides integrity and influences the LNP’s fluidity and shape. As we will see, the choice of this lipid is absolutely critical for controlling reactogenicity.
- The Sterol Component: This is the LNP’s stabilizer, most commonly cholesterol. It fits between the other lipids, managing the particle’s fluidity and structural integrity, much like a chassis frame.
- The PEGylated Lipid (PEG-lipid): This is the “stealth” coating. These lipids have a polyethylene glycol (PEG) chain that extends from the LNP surface, creating a “shield” that prevents the LNP from being immediately cleared by the immune system. This component helps control the particle’s circulation time and size.
The performance of an LNP is dictated by the precise molar ratios of these four ingredients. A tiny change—swapping one helper lipid for another, or altering the PEG-lipid percentage by a single point—can have profound biological consequences.
Taming Reactogenicity: The Power of Helper Lipid Selection
Reactogenicity, the collection of side effects like fever, fatigue, and muscle aches, is a direct result of the body’s innate inflammatory response. This response is triggered by the LNP itself, often leading to a rapid spike in inflammatory cytokines.
The critical insight from recent studies is that this is not an unavoidable cost of LNP delivery. It is a tunable variable.
One of the most dramatic findings comes from comparing LNPs formulated with different helper lipids. In preclinical models, the concentrations of a wide array of inflammatory cytokines and chemokines were measured just three hours after administration.
The results were staggering.
- LNPs formulated with DSPC (a saturated helper lipid) triggered a massive inflammatory storm. Levels of key cytokines—including IFN-α, IFN-β, IL-6, TNF-α, and IL-1β—were hundreds or even thousands of times higher than the control group.
- In stark contrast, LNPs formulated with DOPC (an unsaturated helper lipid) were remarkably quiet. For almost every cytokine measured, the DOPC-based LNPs induced an inflammatory response that was statistically indistinguishable from a simple saline (PBS) injection.
This is a powerful demonstration that reactogenicity can be effectively “engineered out” of a formulation simply by optimizing the helper lipid component. For developers of therapeutics, this means it’s possible to create a delivery system that delivers its payload while remaining “silent” to the innate immune system, opening the door for safer vaccines and, critically, for chronic-dosing mRNA therapeutics where low inflammation is essential.
Boosting Immunogenicity: It’s All About Delivery
Of course, a “silent” LNP is useless if it doesn’t also generate a powerful immune response. This is the other half of the balancing act. We need to tame innate inflammation while promoting the adaptive immune response (antibodies and T-cells).
So, how do LNP components affect the “good” immune response?
The data reveals a fascinating link between where the LNP delivers its message and the strength of the resulting immunity. A deep correlational analysis was performed, linking protein expression in different organs to the eventual immune outcome.
The findings pinpoint the spleen as a critical hub:
- Antibody Generation: Protein expression in the spleen showed a very strong positive correlation (coefficient of 0.78) with the generation of spike-specific IgG antibodies.
- T-Cell Generation: The correlation was even stronger for cellular immunity. Spleen protein expression had an exceptional positive correlation (coefficient of 0.85) with the number of spike-specific CD8+ T-cells.
The message is clear: to get a strong vaccine response, you must design your LNP to efficiently transfect cells in the spleen.
This, too, is a tunable parameter. Studies show that modifying any of the LNP components can modulate the resulting antibody titers:
- Helper Lipids: Just as it affected reactogenicity, the choice of helper lipid (DSPC vs. DOPC vs. DOPE) impacts the final neutralization antibody titers.
- Sterols: Replacing cholesterol with other plant-based sterols like sitosterol, stigmasterol, or campesterol can also be used to fine-tune the antibody response.
- PEGylation: The length of the PEG-lipid chain (e.g., PEG1k vs PEG2k) and its molar percentage (e.g., 0.5% vs 1.5% vs 5.5%) are powerful levers to pull, with different combinations resulting in different levels of neutralizing antibodies.
The Unsung Hero: Formulation Stability
A breakthrough LNP formulation that perfectly balances immunogenicity and reactogenicity is useless if it can’t be manufactured, stored, and shipped. The final, and perhaps most practical, piece of the puzzle is stability.
The LNP’s composition doesn’t just determine its biological function; it determines its physical robustness. To be a viable product, an LNP must maintain its key characteristics—particle size, mRNA encapsulation, and transfection efficiency—over time and at various storage temperatures.
Studies on LNP stability have evaluated formulations at -20°C, 4°C, and 25°C for over 21 days. The results again highlight the critical role of component choice.
For example, an LNP formulated with zero PEG-lipid (PEG0%) was highly unstable. When stored at -20°C, its particle size ballooned, indicating significant aggregation. This formulation also showed a catastrophic drop in mRNA encapsulation, rendering it ineffective.
Conversely, other optimized formulations (like those using DOPC or Stigmasterol) showed excellent stability. They maintained a consistent particle size, kept their mRNA payload securely encapsulated, and, most importantly, retained their in vitro transfection efficiency even after 21 days of storage.
Conclusion: The LNP is a Designable System
The key takeaway is this: the LNP is not a generic “box” for mRNA. It is a highly sophisticated, tunable, and designable system.
The path to the next generation of mRNA medicines lies in this multi-parameter optimization. By carefully selecting each of the four lipid components and their precise ratios, we can:
- Minimize Reactogenicity by intelligently selecting helper lipids to avoid inflammatory pathways.
- Maximize Immunogenicity by designing formulations that target key immune organs like the spleen.
- Ensure Viability by optimizing the formulation for long-term stability across practical storage conditions.
Navigating this complex, multi-variable landscape is the central challenge for all mRNA therapeutic developers. It requires deep expertise in lipid chemistry, biophysics, immunology, and formulation science. As a partner dedicated to the advancement of mRNA technology, we believe that this systematic, data-driven optimization of LNP delivery systems is the key to unlocking the full potential of this revolutionary class of medicine.
Ready to unlock the full potential of your mRNA therapeutics? Creative Biolabs offers comprehensive delivery solutions, including advanced Lipid-based, Polymer-based, and Hybrid vectors, as well as cutting-edge Enveloped Virus-Like Particles. Contact our experts today to design the optimal delivery system for your specific needs.
Reference
Kawaguchi, Yoshino, et al. “Modulating Immunogenicity and Reactogenicity in mRNA-Lipid Nanoparticle Vaccines through Lipid Component Optimization.” ACS nano 19.30 (2025): 27977-28001. CC BY 4.0. https://orcid.org/0000-0002-7265-9221
