Introduction
mRNA is the interlocutor between the genetic code and the protein machine that powers life, part of the dance of molecular biology. The molecule is recently a superstar in the creation of advanced therapies, such as the speedy delivery of COVID-19 vaccines. The promise of mRNA goes beyond vaccines: cancer therapy, protein replacement therapy, and more. The world’s largest biotechnology company, Creative Biolabs, is at the forefront of mRNA research and development, with a suite of services that harness the potential of mRNA to treat diseases. In this blog, we’ll cover the chemical modification of mRNA ends—one of the key factors that determines mRNA stability and therapeutic efficacy—and how Creative Biolabs’ services can aid these efforts.
The Rise of mRNA Therapeutics
mRNA therapies have been an inveterate medical triumph, with the super-fast COVID-19 vaccines. These vaccines already showed what mRNA can do, but there is much more mRNA can do. It allows for the provision of a recipe for any protein that will be generated in the patient’s body by the organic process of protein biosynthesis. It opens up the treatment space only open to human conjecture. We know this best from the hundreds of clinical trials that have already been started on mRNA-based therapies for different ailments. Not only does mRNA technology promise better preventive vaccines (selective vaccines are being developed for influenza, HIV, and Zika, to name but a few), but therapeutic cancer vaccines (personalized, too) that target the patient’s cancer cells with their own immune system. mRNA is also in clinical trials for rare genetic and metabolic disorders that are the result of abnormal body production of proteins. Trials confirming this kind of therapy are phenylketonuria, cystic fibrosis, hemophilia, and many more. Regenerative medicine, cell therapy, or supplying enzymes for targeted genome editing are other uses of mRNA.
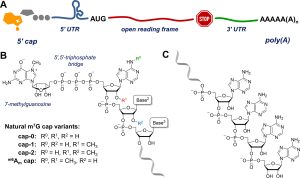
Fig.1 Structure of mRNA: (A) schematic view; (B) mRNA 5′-end (cap); (C) mRNA 3′-end (poly(A) tail).1
The Significance of mRNA Modifications
The article “Chemistic Control of mRNA Caps to Therapeutic Use” by Marcin Warminski et al. discusses the role of the 5′-cap and poly(A) tail in the regulation of mRNA translation and stability. The authors, who have more than 20 years of experience in the field, talk about how synthetic 5′-cap analogs and poly(A) tail modifications make mRNA biocompatible but translationally impairing. Some of the most significant discoveries include mRNA stabilization and translation-enhancing cap analogs and the ability to clinically apply such modifications in mRNA-based anticancer vaccines.
mRNA Stability and Effectiveness
Methods: mRNA Stability and Efficacy Enhancement
The team used in vitro transcription (IVT) and chemical synthesis to synthesize mRNA molecules with modified 5′-caps and poly(A) tails. They also experimented with different cap analogs—S-ARCA, PSL cap , and triazole-containing cap analogs—to see how they affected mRNA stability and translation activity. Poly(A) tail modifications were studied with ATP analogs and poly(A) polymerases (PAPs). The biological activity of the edited mRNAs was monitored through translational efficiency, degradation resistance, and immune evasion assays.
Conclusions: mRNA Therapeutics Are Coming—Where to Next?
The authors concluded that chemical ends on mRNA can greatly increase the therapeutic activity of mRNA. In particular, modifications to the 5′-cap (e.g., -S-ARCA) didn’t just increase mRNA stability, but also translated better and activated the immune system. Poly(A) tail modifications also appeared promising for mRNA stability improvement (though more research is needed to make these modifications work optimally for therapy). In their book, they also make the point that we should continue to study mRNA changes to realize the full potential of mRNA therapies.
Creative Biolabs’ mRNA Services: A Full-Stack Solution
Creative Biolabs has a complete range of services aligned with the studies discussed in the article. We offer mRNA synthesis and modification services like:
1. Personalized mRNAs: With the most recent IVT, we can make mRNA molecules with particular changes to the 5′-cap and poly(A) tail as described in the paper. It enables researchers to evaluate how many different tweaks in their therapeutic usage might work.
2. mRNA modification: We offer all kinds of change services, such as adding synthetic cap analogs or poly(A) tail modifications. Such services can help scientists to ensure mRNA stability and translational efficacy—both critical to successful mRNA therapies.
3. mRNA Delivery Systems: We know how difficult it is to get mRNA into cells, so Creative Biolabs provides LNP formulation services. LNPs have already helped mRNA vaccines to spread and can be designed to capture altered mRNA molecules for better uptake by cells.
4. mRNA Quality Assurance and Testing: We have quality assurance procedures in place that guarantee that our modified mRNA products are 100% pure and functional. That’s essential for preclinical trials and the eventual initiation of mRNA-based therapies.
What mRNA Technology Means More Generally
mRNA vaccines have been so successful, and there’s so much potential in other fields to be used in treatment that there is huge demand for this technology by the pharmaceutical, business, and public sectors. Yet in order for this scaling beyond antiviral vaccines, more mRNA platforms will need to be constructed. The design of therapeutic mRNAs that will become ever more effective and stable and that can be given over and over again without activating the immune system is a task for the future and something that could be addressed using chemical techniques and equipment such as altering the ends of the mRNAs. Another problem for scientists is setting standards for mRNA production, purification, quality control, and biochemical testing. Methods have radically changed in the past few years, and it’s now better understood that cell culture and in vivo assay results can be quite dependent on the purification criteria (particularly double-stranded mRNA abundance) and the targeted cell/biological conditions.
Genetic Disease and mRNA Treatment: The Therapeutic Potential of mRNA.
Genetic diseases will need new methods that improve the durability of mRNA in vitro. For that, chemically enabled poly(A) tails and mRNA circularization are great chess boards. Even though LNPs were a hit for mRNA vaccine delivery, the effective, targeted delivery of mRNA to tissues or cell types is largely underfunded. Spectacular mRNA delivery to specific tissues would allow us to study tissue-specific solutions on the mRNA sequence level and structural levels, such as the cap and poly(A) tail. One thing is for sure: therapeutic mRNA will be there for the taking over the next 10 years; just how far, of course, depends very much on how successful and creative the research community can be in finding new applications and advances to this technology.
The Future of Medicine
mRNA therapies are exploding in pace, and chemical mRNA ends modification is now a growing focus of interest. Creative Biolabs will continue to help this research with our premium services and knowledge. Future horizons: mRNA has great potential to change medicine, and Creative Biolabs will remain central in making it happen. From new vaccines to cancer treatment to rare genetic disorders, mRNA technology and the services provided by organizations such as Creative Biolabs are going to change the face of medicine. The next 10 years will be the decade of therapeutic mRNA, and we’re excited to be part of it, breaking the medicine limit.
Reference:
- Marcin Warminski, Adam Mamot, et al. Chemical Modifications of mRNA Ends for Therapeutic Applications. Accounts of Chemical Research. 2023 56 (20), 2814-2826.
- Distributed under Open Access license CC BY 4.0, without modification.
