The primary characteristic of brain aging is the decline in cognitive functions, including episodic memory, working memory, and the speed of information processing. During the aging process of the brain, the structure of mitochondria, known as the “powerhouses” of brain cells, undergoes significant degenerative changes, and their function markedly declines. However, the regulatory mechanisms behind the changes in mitochondrial structure and function during aging remain unclear. Aging is the most significant risk factor for Alzheimer’s disease, and both cognitive dysfunction and abnormalities in mitochondrial structure and function are key pathological features of Alzheimer’s disease. Yet, how aging leads to the onset and progression of Alzheimer’s disease is not well understood.
On March 8, 2024, the team led by Qiang Liu at the University of Science and Technology of China published an article titled “Aging-induced tRNAGlu-derived fragment impairs glutamate biosynthesis by targeting mitochondrial translation-dependent cristae organization” in the journal Cell Metabolism. The article revealed that a significant amount of glutamate tRNA fragments are produced in the brains during aging and Alzheimer’s disease, which abnormally accumulate within the mitochondria, leading to damage in mitochondrial protein translation and cristae structure, ultimately impairing the synthesis of glutamate and accelerating the pathological process of brain aging and Alzheimer’s disease.
In this study, researchers initially discovered that the expression levels of glutamate tRNA fragments significantly increased during the brain aging process, and this increase was conserved across species. An increase in glutamate tRNA fragments was also detected in the brains of patients with Alzheimer’s disease. Further research indicated that the generation of glutamate tRNA fragments depends on angiogenin (ANG): ANG aberrantly relocates from the nucleus to the cytoplasm during aging and cleaves glutamate tRNA.
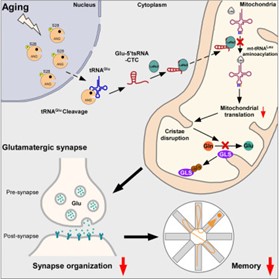
Researchers utilized molecular hybridization and immunofluorescence techniques to find that glutamate tRNA fragments abnormally accumulate in the mitochondria of glutamatergic neurons. Through RNA-pull down assays and proteomics, the binding protein of glutamate tRNA fragments was identified as mitochondrial leucyl-tRNA synthetase (LaRs2). Biochemical experiments and transmission electron microscopy revealed that glutamate tRNA fragments and mitochondrial leucyl-tRNA competitively bind to LaRs2, disrupting the aminoacylation of mitochondrial leucyl-tRNA and the translation process of mitochondrial proteins, further damaging the mitochondrial cristae structure and the glutaminase-dependent synthesis process of glutamate, thereby accelerating the process of brain aging.
Designing antisense oligonucleotides targeting this tRNA fragment and injecting them into the brains of aging mice were found to significantly alleviate the learning and memory impairments in aging mice by restoring protein translation in the mitochondria of glutamatergic neurons, cristae structure, and brain glutamate levels. These research findings further support that glutamate tRNA fragments are significant risk factors for accelerating brain aging, offering new therapeutic targets for slowing down age-related cognitive impairments.
In summary, this study revealed that glutamate tRNA fragments, which abnormally accumulate in brain aging and Alzheimer’s disease, by damaging the mitochondrial protein translation process and cristae structure, inhibit the synthesis of glutamate, thereby accelerating the pathological processes of brain aging and Alzheimer’s disease. This research is of significant importance for understanding the pathogenesis of brain aging and Alzheimer’s disease, revealing a novel role of tRNA fragments in brain aging, and proposing new therapeutic approaches for delaying cognitive decline.
Equipped with state-of-the-art facilities and highly experienced staff, Creative Biolabs has integrated high-quality mRNA-based services that span the entire development chain from discovery to commercialization, including but not limited to:
- mRNA for Immunotherapy
- mRNA for Gene Therapy
- mRNA for Protein Replacement Therapy
- mRNA for Regenerative Medicine
- Therapeutic Antibody-Coding mRNA
- mRNA Pharmacology Optimization
- mRNA Vaccine Development
Reference:
Li, Dingfeng, et al. “Aging-induced tRNAGlu-derived fragment impairs glutamate biosynthesis by targeting mitochondrial translation-dependent cristae organization.” Cell Metabolism (2024).
