Researchers from University College London, King’s College London, and Moderna have published a paper in the journal Science Translational Medicine titled: mRNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical Research paper by Argininosuccinic Aciduria.
The study developed an mRNA therapeuti that delivers human arginine succinate lyase mRNA (hASL-mRNA) through lipid nanoparticles (LNPs), which effectively treated argininosuccinic aciduria, a rare liver genetic disease, in a mouse model, corrected glutathione metabolism defects, and restored urea production, demonstrating the potential application value of mRNA technology in the treatment of human diseases.
The research team aims to conduct human clinical trials in the coming years, and Moderna is already conducting clinical trials of mRNA therapeutics for other inherited metabolic diseases, including propionic acidemia and methylmalonic acidemia.
In this study, the research team explored the feasibility of using mRNA technology to treat arginine succinituria. This therapy uses lipid nanoparticles (LNPs) to deliver mRNA (hASL-mRNA) of human arginine succinate lyase to ameliorate abnormal glutathione metabolism and chronic liver disease caused by ALS deficiency. They tested the treatment on 31 mice, including juvenile mice with disease at birth, and adult mice with advanced disease, as a salvage treatment for arginine succinitururia, and they also used the same number of untreated mice as a control group.
During the treatment, mice underwent positron emission tomography (PET) scans to track the correction and treatment effect of mRNA therapy on glutathione regulation in a non-invasive manner.
The results showed that hASL-mRNA therapy corrected the phenotype of neonatal and adult ASL-deficient mice, enhanced urea production, and ameliorated the fatal consequences of arginine succinuria. All mice born with the disease died within the first two weeks of life, while those who received mRNA therapy at birth survived for more than three months. In addition, six of the seven elderly sick mice treated with mRNA as a salvage treatment survived, while all untreated aged mice died.
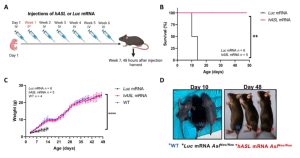
Figure 1. Therapeutic effects of hASL-mRNA therapy in mice with nascent diseases1
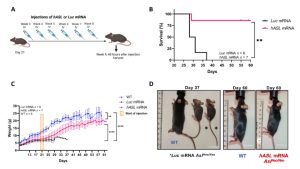
Figure 2. Therapeutic effect of hASL-mRNA therapy in adult mice with disease1
For mice, the benefits of each mRNA treatment lasted only about seven days, with weekly treatments over a period of up to eight weeks. But the research team expects that mRNA will allow for longer treatment intervals when used in the treatment of human patients.
According to the research team, the study demonstrates the unprecedented therapeutic potential of mRNA technology for genetic diseases that are currently untreatable, especially liver diseases. The team’s goal is to further apply this approach to other inherited liver diseases and advance mRNA therapeutics into human clinical trials.
Dr. Paolo Martini, Chief Scientific Officer at Moderna’s International Therapeutic Research Center, said the collaboration demonstrates the synergy between academia and industry to explore the use of mRNA technology to combat rare diseases and potentially bring treatments for serious and debilitating diseases like arginine succinuria.
Our Platform for mRNA Therapeutics
- mRNA for Immunotherapy
- mRNA for Gene Therapy
- mRNA for Protein Replacement Therapy
- mRNA for Regenerative Medicine
- Therapeutic Antibody-Coding mRNA
- mRNA Pharmacology Optimization
- mRNA Vaccine Development
Reference:
- Gurung, Sonam, et al. “mRNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical argininosuccinic aciduria.” Science translational medicine 16.729 (2024): eadh1334.
