In recent years, mRNA technology has emerged as a transformative force in biomedical science, revolutionizing how we approach disease prevention and treatment. While its initial breakthrough with COVID-19 vaccines captured global attention, the true promise of this technology extends far beyond infectious diseases. From personalized cancer therapies to groundbreaking treatments for genetic disorders, mRNA’s versatility continues to redefine the boundaries of modern medicine. This article explores the latest advancements in mRNA research, focusing on its expanding applications, technological innovations, and the challenges that lie ahead.
The Evolution of mRNA Technology
mRNA, or messenger RNA, serves as a molecular blueprint that instructs cells to produce specific proteins. Unlike traditional vaccines or therapies, which often rely on live pathogens or invasive interventions, mRNA-based approaches introduce synthetic RNA strands into cells, enabling them to generate therapeutic proteins in situ. This mechanism offers several key advantages, including rapid development timelines, scalability, and minimal risk of genomic integration.
The success of COVID-19 vaccines highlighted mRNA’s potential, but researchers are now leveraging this platform for a broader range of applications. For instance, mRNA can be engineered to encode tumor-specific antigens, immune modulators, or even enzymes to correct genetic defects. This adaptability has sparked intense interest in its use for cancer immunotherapy, rare disease treatment, and gene editing.
mRNA in Oncology: A Paradigm Shift in Cancer Treatment
One of the most exciting frontiers for mRNA technology is personalized cancer vaccines. These vaccines are designed to target unique mutations (neoantigens) present in a patient’s tumor, triggering a targeted immune response. In 2024, multiple clinical trials demonstrated the efficacy of such vaccines. For example, a Phase 2b study evaluating a personalized mRNA cancer vaccine in melanoma patients showed a 44% reduction in recurrence risk when combined with immunotherapy. Similarly, a Phase 1 clinical trial for pancreatic cancer reported that patients who responded to the vaccine experienced significantly longer relapse-free survival.
Beyond personalized approaches, mRNA vaccines are also being developed for shared tumor antigens. These “off-the-shelf” vaccines could streamline production and reduce costs, making them accessible to a broader patient population. In China, several mRNA-based tumor vaccines targeting HPV, EBV, and other oncogenic viruses have entered clinical trials, reflecting the global momentum in this field.
Another innovative application is in vivo CAR-T therapy, where mRNA-LNP (lipid nanoparticle) formulations directly deliver CAR (chimeric antigen receptor) coding sequences to immune cells. This eliminates the need for ex vivo cell manipulation, potentially lowering costs and treatment timelines. Preclinical studies have shown promising results in lymphoma and autoimmune disease models, with clinical trials expected to begin in 2025.
Tackling Rare Diseases and Genetic Disorders
mRNA technology is also making strides in treating rare and genetic diseases. Protein replacement therapy, which delivers mRNA encoding missing or defective proteins, has shown efficacy in conditions like propionic acidemia. A 2024 trial revealed that patients treated with an mRNA-based therapy experienced a 70% reduction in disease-related events. Similarly, studies on DSC2-deficient cardiomyopathy demonstrated that a single injection of modified mRNA restored cardiac function in preclinical models, offering hope for hereditary heart conditions.
For genetic disorders, mRNA can be combined with CRISPR-Cas9 gene editing tools. Non-viral delivery systems, such as LNPs, enable precise targeting of tissues like the liver, where they correct mutations causing diseases like transthyretin amyloidosis. Clinical trials of such therapies have reported over 90% reduction in disease-causing proteins with minimal side effects.
mRNA Delivery Service
Creative Biolabs offers professional mRNA delivery services. It provides a variety of vector development options:
- For lipid-based vectors, services include LNP formulation, encapsulation efficiency assessment, and in vitro and in vivo delivery efficiency testing.
- Regarding polymer-based vectors, it offers polymer design and synthesis, formulation optimization, and biocompatibility studies.
- For hybrid vectors, services cover customizing vector components, ensuring high gene delivery efficiency, and rigorous quality control.
These services are highly customizable to meet specific research and therapeutic needs.
Advancements in Delivery Systems
The efficacy of mRNA therapies hinges on efficient delivery. LNPs, the gold standard for COVID-19 vaccines, continue to evolve. New formulations enhance stability, reduce immunogenicity, and improve tissue specificity. For example, antibody-conjugated LNPs have been developed to target specific cell types, such as B cells in autoimmune diseases.
Alternative delivery methods, like lipid-polymer hybrid nanoparticles (LPPs), are also gaining traction. These systems offer prolonged mRNA release and better protection against degradation, as demonstrated in preclinical studies of personalized cancer vaccines. Additionally, research into inhalable mRNA formulations for respiratory diseases and topical applications for skin conditions is underway, expanding the technology’s versatility.
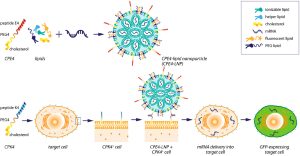
Fig. 1 Schematic of delivery of non-viral lipid nanoparticles.1
Overcoming Challenges: Stability and Accessibility
Despite its promise, mRNA technology faces hurdles. The inherent instability of RNA molecules requires stringent storage conditions, often at ultra-low temperatures, limiting accessibility in resource-poor settings. To address this, scientists are optimizing mRNA sequences (e.g., introducing pseudouridine modifications) and exploring lyophilization techniques to improve shelf-life. Regulatory bodies like the FDA and NMPA are also updating guidelines to streamline the development of thermostable mRNA vaccines.
Another challenge is immune tolerance. While LNPs minimize inflammation, repeated mRNA administration can trigger anti-lipid or anti-RNA immune responses. Strategies to mitigate this include co-delivering immunosuppressive molecules or engineering LNPs with stealth properties.
The Road Ahead: Integration with AI and Multidisciplinary Approaches
The future of mRNA technology lies in its integration with artificial intelligence (AI) and multidisciplinary research. AI algorithms are already being used to predict neoantigens, design optimized mRNA sequences, and accelerate vaccine development.
Combination therapies—such as mRNA vaccines with checkpoint inhibitors or chemotherapy—are also showing synergistic effects. In pancreatic cancer, the combination of an mRNA vaccine and PD-1 blockade led to partial tumor regression in advanced cases. Similarly, mRNA-based gene editing therapies are being paired with protein replacement strategies to address complex genetic disorders.
mRNA technology has transcended its origins as a vaccine platform, evolving into a versatile tool for precision medicine. From personalized cancer therapies to gene editing and rare disease treatments, its applications are as diverse as they are groundbreaking. While challenges like stability and scalability persist, ongoing innovations in delivery systems, AI integration, and clinical trial design are paving the way for a new era of patient-centered care. As researchers continue to push boundaries, the true potential of mRNA—unlocking cures for previously untreatable diseases—grows ever closer to realization.
References
Aschmann, Dennis, Renzo A. Knol, and Alexander Kros. “Lipid-based nanoparticle functionalization with coiled-coil peptides for in vitro and in vivo drug delivery.” Accounts of Chemical Research 57.8 (2024): 1098-1110. https://doi.org/10.1021/acs.accounts.3c00769
